Identification of fracture mechanisms is a major stage in performing Root Cause Failure Analysis (RCFA). At ESR Technology’s National Centre of Tribology, our team of expert engineers carry out metallurgical failure analysis on a wide range of materials across a variety of industries.
An example is the increasing use of high-strength steel for bolting and fixtures. High-strength steels are generally obtained by quenching from the austenite phase to form martensite and subsequently tempering can obtain the required metal properties.
Tempered martensite has relatively low ductility which increases the risk of hydrogen embrittlement (HE), also known as hydrogen-assisted cracking or hydrogen-induced cracking (HIC). Hydrogen atoms are small and can permeate solid metals. Once absorbed, hydrogen lowers the stress required for cracks in the metal to initiate and propagate, resulting in embrittlement. In general, materials with increased hardness, such as high-strength steel. Some nickel, titanium and cobalt alloys are also more susceptible to hydrogen embrittlement.
The precise mechanism of HE is complex, and several theories and mechanisms have been proposed such as an internal pressure theory, reduction in lattice cohesion, reduction of surface energy, brittle crack tip theory, hydride-induced cracking, and localised slip model. In reality, several mechanisms likely work in synergy, however for hydrogen embrittlement to occur the following are required:
- HE must start with hydrogen atoms diffusing through the metal.
- Increased temperature increases the mobility of the hydrogen atoms which can drive the hydrogen further through the metal. Hydrogen, however, will still diffuse through a metal at low temperature, as long as there is a concentration gradient.
- The hydrogen atoms will tend to collect at discontinuities within the steel matrix. The most obvious discontinuities are grain boundaries and inclusions; the grain boundaries become embrittled, significantly reducing the mechanical properties.
- Atomic hydrogen can form molecular hydrogen which increases the internal pressure. The internal pressure can further weaken the steel and if sufficient hydrogen is present produce cracks (HIC).
Hydrogen embrittlement can occur during various manufacturing operations, or, through operational use. Any situation where a metal may come into contact with atomic or molecular hydrogen. Processes including electroplating, cathodic protection, phosphating, pickling, and arc welding can promote HE.
HE is a well-documented problem in bolts and fasteners that are electroplated i.e. galvanising. This risk can be eliminated by baking the bolts at 200C for 24 hours after coating so that the hydrogen can diffuse out of the structure.
Another mechanism of hydrogen introduction is through corrosion, Figure 1. Chemical reactions with acids or other chemicals (notably hydrogen sulphide in sulphide stress cracking, or SSC, a process of importance for the oil and gas industries). However, corrosion of high-strength steel is the most insidious. During the corrosion process, the surface of the steel is attacked, this can occur in five stages:
- General surface corrosion
- Formation of U-shaped corrosion pits
- U-shaped pits develop into V-shaped pits.
- Cracks form at the base of the pits
- Cracks propagated through the material until failure occurs.
HE mechanisms can be identified by Scanning Electron Microscopy (SEM). Steels that fail under tensile loading will possess either a brittle mode (Figure 2) or fail in a ductile manner (Figure 3). A brittle fracture occurs when grains cleave apart resulting in relatively flat facets on the surface of the fracture. A ductile fracture has a cup and cone or dimpled appearance as a result of the material elongating before breaking. Often fracture surfaces can exhibit both types of fracture and are described as mixed mode.
HE however shows different fracture characteristics; the embrittled grain boundaries fail preferentially resulting in an intergranular fracture where the Individual grains can be seen with a narrow gap around the grains (Figure 4).
If you require metallurgical support or failure analysis or have any questions for our NCT Team, please contact us by emailing us at info@esrtechnology.com
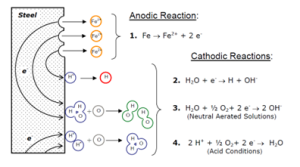
Figure 1: Chemical reactions as a result of aqueous corrosion on a steel surface with the formation of atomic Hydrogen.

Figure 2: Brittle cleavage fracture.

Figure 3: Cup and cone ductile fracture.
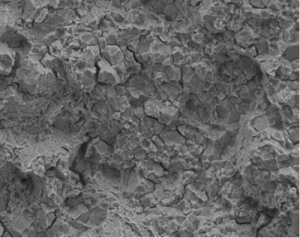
Figure 4: Intergranular fracture, grain boundaries visible.