When we examine bearings as part of a failure investigation or as a routine assessment, grease analysis is an important step, revealing issues like water ingress, debris contamination, or degradation of the base oil. We use a suite of tools to assess the condition of the grease from measurement of its consistency to elemental analysis by atomic emission spectroscopy.
One method that gives a variety of detailed information about the grease chemistry is FTIR, which works by passing a rainbow of infra-red light through a sample and measuring the absorbed frequencies. Different absorption frequencies identify different chemical bonds present in the sample. In this article, we will explore some of the insights that can be gleaned from this technique.
1. Base oil chain length
In most greases, the base oil is a long chain alkane, such as cetane, shown below.
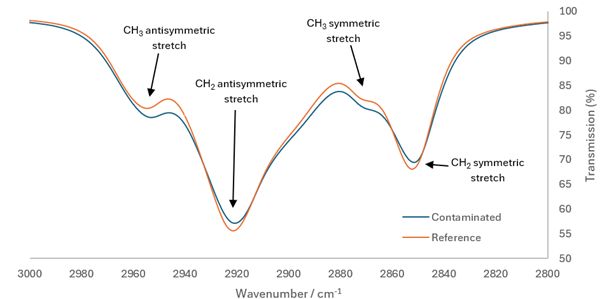
Such molecules contain two different kinds of C-H bonds: those in -CH2– groups and those in -CH3 groups, with slightly different infra-red signatures. The ratio of the absorption intensities for CH2 and CH3 can be calculated, and oil mixtures containing more longer chain alkanes will have a higher ratio. An increase in this ratio is often caused by evaporative loss of the lighter fractions due to elevated operating temperature, though polymerisation is also possible. To explore this further, we use mass spectrometry to definitively separate out the different molecular fragments.
2. Water content
Large amounts of water in a grease can be identified by thermogravimetric analysis, or even more simply by the crackle test, wherein the operator listens for the crackling sound as water boils out of a heated sample of grease. However, many greases contain corrosion inhibitors, which can absorb substantial amounts of water by reacting with them; the ingress of water can then be missed by TGA or the crackle test.
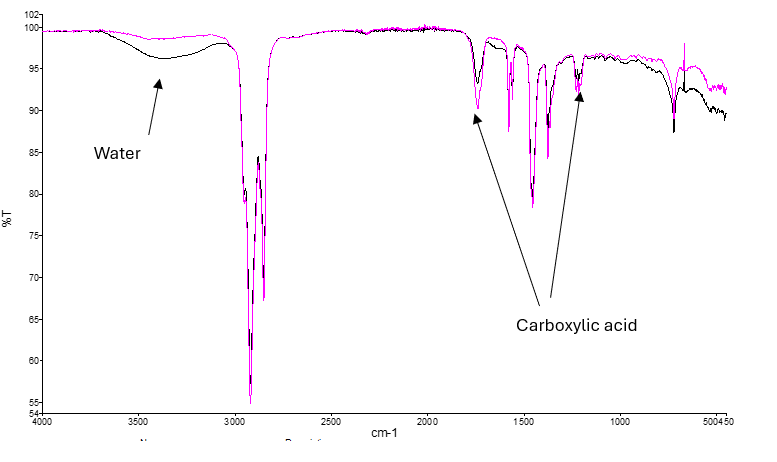
Using FTIR, we can detect the reaction products, often carboxylic acids. To generate the spectra below, water was mixed into a grease sample. Spectra were recorded immediately after mixing (black), and after around ten minutes (magenta). The reaction is clearly visible, consuming water and generating carboxylic acid.
3. Oxidation
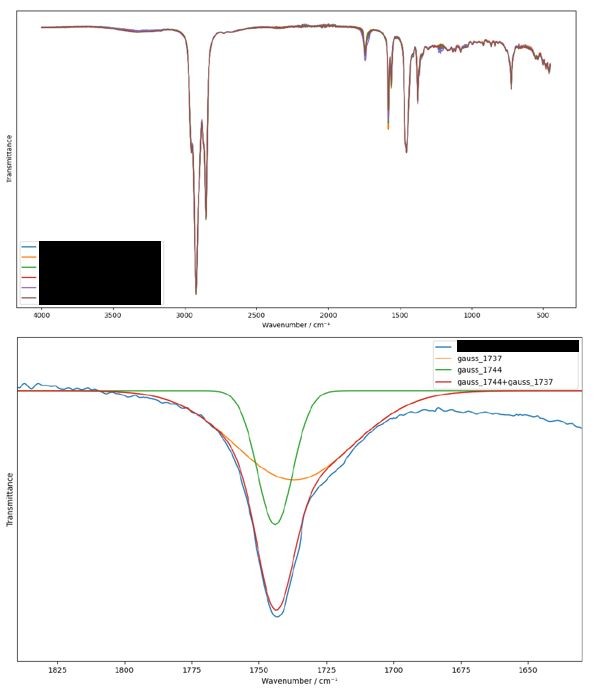
When subject to high temperatures, oils oxidise over time, forming alcohols, aldehydes and ketones, and carboxylic acids. The identification of oil oxidation by FTIR requires careful analysis, as the signature reaction products overlap with those of the aforementioned corrosion inhibitors. Mathematical techniques can be used to analyse overlapping signals and distinguish oxidation from hydration products.
We have developed our own custom Python toolkit for these sorts of quantitative analyses. In the spectra below, we separated out contributions from aldehydes and carboxylic acids and corelated them with other markers in the spectra to assess the degree of oxidation of the base oil.
4. Get in touch for more!
At ESR we leverage our expansive suite of analytical equipment, combined with an interdisciplinary team spanning tribology, chemistry, and mechanical engineering to deliver insights you won’t find elsewhere. We can combine grease analysis by various techniques with a detailed physical examination of the lubricated components, statistical analysis of past failures, and engineering analysis and review of designs, to give robust, evidence-driven solutions to reliability issues, catastrophic failures, and more.
Send us an enquiry via our contact page or email us on info@esrtechnology.com